About the chemical structure:
| Chemical name: | Lead(II)-antimonate |
| Formula: | Pb(SbO3)2 or Pb(SbO4)2 |
| Crystal system: | Bindheimite: Isometric - Gyroidal (at Mineralogy Database) |
| Refractive index: | 2.01 - 2.28 |
Color:
| Color Index (C.I.) | PY 41 |
How can you identify Naples yellow?
Imaging:
IRFC: white
OM: particles are homogenous and appear very fine and finely divided, similar to what you would expect from a synthetic pigment.
No crystalline form can be detected even at high magnification. Particles are usually between 1-5μm in size.
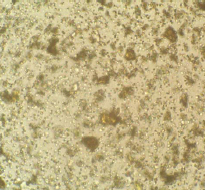
Microscopic appearance at x500 mag
Analytics:
It's identified by means of FTIR and Raman. Confirmed by microchemical tests for lead and antimony.
Raman spectra: University College London;
FTIR spectra: IRUG
Usage and handling:
| Permanence: | Toxicity: |
|---|---|
Lightfast: excellent. Degradation processes: chemically it is quite stable and is little affected by alkalis, dilute or concentrated nitric or hydrochloric acids. It fuses only at high temperature but turns darkbrown permanently. In oil Naples yellows is quite permanent, so far as light is concerned, but it is liable to be darkened, like other lead compounds, by air containing hydrogen sulfide. In water-colour painting Naples yellow is quite inadmissible, for it blackens rapidly, but irregularly, in the presence of mere traces of sulphur compounds. |
very toxic. Contains lead. MSDS: Kremer |
Literature:
O’Shea, M. L., A Historical and Chemical Analysis of the Artists’ Pigment Naples Yellow (PhD Thesis), Reed College 1981
Artists’ Pigments A Handbook of Their History and Characteristics, Vol. 1, L. Feller, Ed, Cambridge University Press, London 1986, p. 219-254
(intro) - Cadmium yellow/red - Chrome yellow - Cobalt yellow - Indian yellow - Lead-tin yellow - Lemon yellow - Naples yellow - Orpiment - Yellow ochre

