About the chemical structure:
| Chemical name: | Basic Copper acetate |
| Formula: | |
| Refractive index: | alpha = 1.53; gamma = 1.56 |
Color:
| Color Index (C.I.) | PG 20 |
How can you identify Verdigris?
Imaging:
UVF: no
IRFC: blue
OM: Intense green crystals of different shapes and sizes. Particles can vary greatly depending upon the method of manufacture, for instance well-crystalised verdigris particles appear like shards of pointed needles; Whereas, particles that have not undergone crystalisation may appear like transparent grains. Due to its varying states, particles are difficult to distinguish, their distinct green colour and resinous quality are the main clues to its identity. Particles exhibit pleochroism, some becoming colourless upon rotation at crossed polars, although some may appear to turn a deep bluish green. Particles are usually 1-30μm in size.
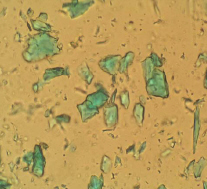
Microscopic appearance at x500 mag
Analytics:
It's identified by means of FTIR and Raman.
Raman spectra: University College London;
FTIR spectra: IRUG
Usage and handling:
| Permanence: | Toxicity: |
|---|---|
Lightfast: good. Degradation processes: verdigris is fugitive and reacts upon contact with hydrogen sulphides turning black. Infamous for being destructively reactive, verdigris degrades cellulosic materials and parchment.
The green areas painted with verdigris show up in the recto of this page because the acetic acid in the pigment when freed by moisture reacts with the parchment.
|
moderately toxic. Verdigris contains cooper, which is rated as toxic from prolonged exposure if inhaled or ingested. Care should be used in handling the dry powder pigment to avoid inhaling the dust. MSDS: Kremer |
Literature:
Kühn, H., Grünspan und seine Verwendung in der Malerei, Farbe und Lack, 70, 1964, p. 703-711
Schweizer, F. und Mühletaler, B. Einige Grüne und Blaue Kupferpigmente, Farbe und Lack, 74 1968, p. 1159-73
Artists’ Pigments. A Handbook of Their History and Characteristics, Vol. 2: A. Roy (Ed.) Oxford University Press 1993, p. 131-147
(intro) - Cobalt green - Copper resinate - Emerald green - Green earth - Malachite - Verdigris - Viridian


