About the chemical structure:
| Chemical name: | Iron(III)-oxide, partly hydrated + manganese oxide partly hydrated + humic acids |
| Formula: | Fe2O3 (· H2O) + MnO2·(n H2O) + humic acids
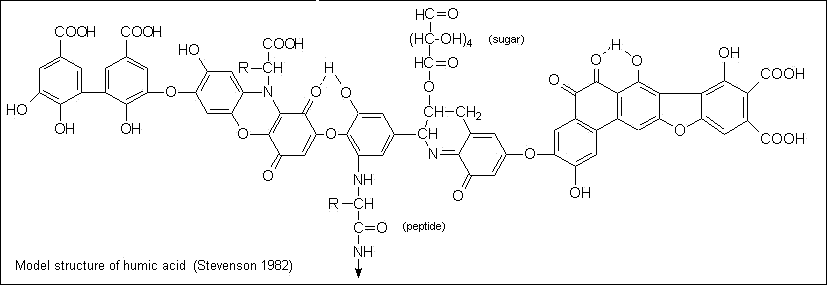
Humic acids are thought to be complex aromatic macromolecules with amino acids, amino sugars, peptides, aliphatic compounds involved in linkages between the aromatic groups. The hypothetical structure for humic acid, shown in figure, contains free and bound phenolic OH groups, quinone structures, nitrogen and oxygen as bridge units and COOH groups variously placed on aromatic rings. |
| Refractive index: | n/a |
Color:
| Color Index (C.I.) | PBr 8 |
How can you identify Van Dyke brown?
Imaging:
IRFC: black
OM: particles appear more opaque and crystalline than ochres and umbers. Particles look more like flakes and some appear fibrous. Particles are usually 1-50μm in size.
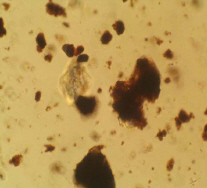
Microscopic appearance at x500 mag
Analytics:
It's identified by means of FTIR. The pigment appears to dissolve in oil and in varnish to stain it as a result it is difficult to identify in such mediums.
n/a
-->FTIR spectra: IRUG
Usage and handling:
| Permanence: | Toxicity: |
|---|---|
Lightfast: good. Degradation processes: it fades on exposure to strong light and develops a cold, grey tone. Does not solidify in mixtures with oil, but is stable in varnish solutions. More permanent in oil than in water colour. Partially transparent in oil so was often used for staining woods and for glazes on paintings. |
non toxic. However one of its components, manganese oxide, is moderately toxic and care should be used in handling the dry powder pigment so as not to inhale the dust. MSDS: Natural pigments |
Literature:
Artists’ Pigments. A Handbook of Their History and Characteristics, Band 3: E.W. Fitzhugh (Ed.) Oxford University Press 1997, p. 157-190