About the chemical structure:
| Chemical name: | Lead(II,IV)-oxide |
| Formula: | Pb3O4 |
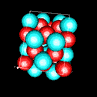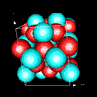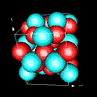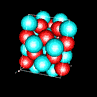
| 3D model: |
red = oxygen, light blue = lead |
| Crystal system: | tetragonal |
| Refractive index: | 2.42 |
Color:
| Color Index (C.I.) | PR 105 |
How can you identify Red lead?
Imaging:
UVF: dark red
IRFC: yellow-brown
OM: Tiny homogenous particles often clumped together. Particles tend not to be very characteristic. Crystals are usually 1-50μm in size. High refractive index but only slightly birefringent.
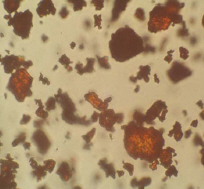
Microscopic appearance at x500 mag
Analytics:
It's identified by means of Raman. Chemically reactive turning brown with nitric or acetic acid resulting in the formation of brown lead oxide. Hydrochloric acid turns it white (lead chloride) and sulphides blacken it.
Raman spectra: University College London;
Usage and handling:
| Permanence: | Toxicity: |
|---|---|
Lightfast: poor. Degradation processes: red lead is liable to discoloration in the presence of air pollutants such as hydrogen sulfide. It reacts upon some pigments that contain free amounts of sulfur, such as poorly made cadmium yellows, for example. It should not be used in watercolor painting, as it may darken when exposoed to humidity and light. When mixed with oil, it is fairly permanent. It is best suited as an oil color or in encaustic paint. It has a good reputation in tempera painting. It has been observed to lighten in fresco painting due to the formation of lead sulfate from its interaction with moisture and the atmospheric pollutant of hydrogen sulfide. Initially orange-red in colour it is photoxidized to a light pink in the sun or a brownish red depending upon the environmental influences. Browning has more notably occurred when it has been applied in water colour or tempera medium. The PbO2 functions as an oxidising agent within the whole structure of PbO2 . 2PbO resulting in the change in hue over time. |
very toxic. Red lead is highly toxic, and utmost care should be used in handling the dry powder pigment to avoid inhaling the dust or ingesting the pigment in any form. MSDS: Natural pigments |
Literature:
Artists’ Pigments A Handbook of Their History and Characteristics, Vol. 1, L. Feller, Ed., Cambridge University Press, London 1986, p. 109-139
Dunn, E.J. Red Lead in Pigment Handbook, vol.1, ed. T.C. Patton, New York 1973, p. 837-42