About the chemical structure:
| Chemical name: | Basic lead(II)-carbonate |
| Formula: | 2 PbCO3· Pb(OH)2 |
| Crystal system: | Trigonal (at Mineralogy Database) |
| Refractive index: | Uniaxial (-), e = 1.94, w = 2.09 |
Color:
| Color Index (C.I.) | PW 1 |
How can you identify Lead white?
Imaging:
UVF: pale white
IRFC: white
OM: Particles exhibit high birefringence. Polarisation shows bright white crystals, a few may appear to be orange orred.
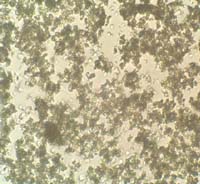
Microscopic appearance at x500 mag
Analytics:
It's identified by means of FTIR and Raman.
Raman spectra: University College London;
FTIR spectra: IRUG
Usage and handling:
| Permanence: | Toxicity: |
|---|---|
lightfast: excellent Degradation processes: its surface is blackened on contact with sulphides in the air causing the chemical reaction of lead carbonate to black lead sulphide. The lead hydroxide part of lead white molecule is able topartially 'saponify' linseed oil to form with it a lead soap called lead linoleate. This fact has been used to explain why lead white in oil forms has such a hard and porous paint film. It is also a given reason for the transparency of aged lead white paint films. |
toxic. Cerussite is toxic and care should be used in handling the dry powder pigment so as not to inhale its dust, as well as the pigment dispersed in medium. That is why you never see this color in water dilutable paints; it would be too dangerous to use. However, when incorporated into an oil paint and used in a conventional manner with routine hygienic precautions, it can be used safely. Just make sure to keep your hands and clothing clean and free from solvent or painting medium that has been mixed with this color. A little soap and water goes a long way. After all, oil painters have been using lead white for hundreds of years without ill effects, simply by using common sense precautions. MSDS: Kremer |
Literature:
Kühn, H., Bleiweiss und seine Verwendung in der Malerei I. und II., Farbe und Lack, 73, 1967, p. 99-105 and 209-213
R. J. H. Clark and P. J. Gibbs, Raman Microscopy of a 13th Century Illuminated Text, Analytical Chemistry (vol 70, p 99A)Artists’ Pigments. A Handbook of Their History and Characteristics, Vol. 2: A. Roy (Ed.) Oxford University Press 1993, p. 67-81

