Milk fat is comprised mostly of triglycerides, with small amounts of mono- and diglycerides, phospholipids, glycolipids, and lipo-proteins. The trigylcerides (98% of milkfat) are of diverse composition with respect to their component fatty acids, approximately 40% of which are unsaturated fat firmness varies with chain length, degree of unsaturation, and position of the fatty acids on the glycerol. This is a typical breakdown of fatty acids in butter:

Flavorful fatty acids play an important role in the flavor of butter and are present at varied concentrations. Although long-chain fatty acids are present at higher concentrations in butter, they do not make a significant contribution to flavor. Short-chain fatty acids (SCFA), on the other hand, do play an important role in butter's flavor.
Typically, SCFA are found in the serum portion of butter (aqueous solution of all non-fat components) where their flavor potential is stronger. They occur below their Flavor Threshold Value (FTV): the minimum concentration level below which aroma or taste is imperceptible. Despite low concentrations, SCFA react in a synergistic and additive manner to provide characteristic flavors found in butter. Butyric acid is the most widely known and most potent SCFA and is attributed to providing intensity to fatty acid-type flavors associated with butter. Butter also contains a variety of fatty acid precursors of 4-cis-heptenal, a compound which provides butter with a creamy flavor.
It is a curious feature of fats that once melted, they have to be cooled to well below their melting point to resolidify them. Butter, for example, melts at about 35 °C (96 °F) but has to be cooled to about 23 °C (73 °F) to solidify it.
Milkfat from cows fed diets higher in stearic acid produced softer butter than milkfat from cows fed diets higher in palmitic acid. The change in butter softness was associated with changes in fatty acid and triglyceride structure of the milkfat.
Butyric acid
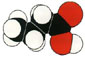 Butyric acid, also called butanoic acid (CH 3CH 2CH 2CO 2H), is a fatty acid occurring in the form of esters in animal fats and plant oils. As a glyceride, it makes up 3–4 percent of butter ; the disagreeable odour of rancid butter is that of butyric acid resulting from hydrolysis of the glyceride. The acid is of considerable commercial importance as a raw material in the manufacture of esters of lower alcohols for use as flavouring agents; its anhydride is used to make cellulose acetatebutyrate, a useful plastic.
Butyric acid, also called butanoic acid (CH 3CH 2CH 2CO 2H), is a fatty acid occurring in the form of esters in animal fats and plant oils. As a glyceride, it makes up 3–4 percent of butter ; the disagreeable odour of rancid butter is that of butyric acid resulting from hydrolysis of the glyceride. The acid is of considerable commercial importance as a raw material in the manufacture of esters of lower alcohols for use as flavouring agents; its anhydride is used to make cellulose acetatebutyrate, a useful plastic.
Butyric acid can be artificially manufactured by aerial oxidation of butyraldehyde. It is a colourless liquid, soluble in water and miscible with common organic solvents; it freezes at -4.26° C (24.33° F) and boils at 163.53° C (326.35° F). An isomer, isobutyric acid (CH 3)2CHCO 2H, or 2-methylpropanoic acid, is found both in the free state and as its ethyl ester in a few plant oils. It is commercially less important than butyric acid. It is generally similar to butyric acid; it freezes at -46.1° C (-51° F) and boils at 153.2° C (307.8° F).
Linoleic Acid
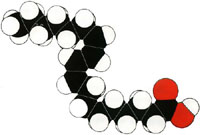 Linoleic Acid is the principle fatty acid in many vegetable oils, including cottonseed oil, soybean oil, and corn oil. It is also abundant in rapeseed oil (from members of the mustard family, Brassicaceae) and is used in the manufacture of margarines, shortening, and salad and cooking oils.
Linoleic Acid is the principle fatty acid in many vegetable oils, including cottonseed oil, soybean oil, and corn oil. It is also abundant in rapeseed oil (from members of the mustard family, Brassicaceae) and is used in the manufacture of margarines, shortening, and salad and cooking oils.
Triglycerides built from linoleic acid are oils because of the double bonds in their chains of carbon atoms. Since margarine and shortening manufacturers want a soft solid, they bubble hydrogen through the oil in the presence of a nickel catalyst. This hydrogenation process brings about several changes, including the partial saturation of the carbon chains as hydrogen atoms attach to carbon atoms that were originally joined by double bonds. The replacement of carbon-carbon double bonds with single bonds allows the carbon chains to become flexible. As a result, the molecules can pack together more closely and the oil is converted to a fat. The hydrogenation process is stopped sooner if the oils are destined to become softer (tub) margarines.
The elmination of the double bonds during the hydrogenation also reduces the likelihood of attack by oxygen, so that the fat remains fresh longer. Nasty-smelling molecules are removed by passing superheated steam through the molten fat. This also rmoves the molecules responsible for color, so carotenes of various kinds are added to restore a butterlike appearance. The odor of butter is simulated by adding butanedione. The flavor is enhanced and sharpened by emulsifying the fats with skimmed milk that has been cultured with bacteria that produce lactic acid. The nutritional value is improved by the addition of vitamins A and D. And finally, natural surfactant molecules (lecithins, which are triglyceride like substances with one side chain containing a phosphatelike group) are added to ensure that the entire conccoction hangs together.
