A page from the "Causes of Color" exhibit...
What causes the color in blue diamonds and in LED lamps? (doped semiconductors)

In the 13th century, France’s Louis IX must have recognized the beauty and timelessness of diamonds, since he decreed that the gems could be owned only by the King. Had he been prescient, he might have known that the renowned French Blue Diamond would be stolen from the French Royal Treasury in the late 18th century, then re-cut to become what is today revered as the Hope Diamond. Yet he never could have imagined that, one day, that stunning blue diamond would have something in common with semiconductors, LED lamps, and lasers.
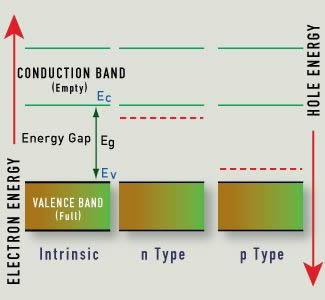
Like metals, semiconductors appear colored because of the interaction of incoming light with valence electrons. Unlike electrons in metals, electrons in semiconductors are bound to a particular atom unless excited. The energy emitted when an electron drops from the conduction band to the valence band for certain semiconductors corresponds to electromagnetic radiation in the visible light spectrum. The band gap determines the color of light we perceive.
Semiconductors are immensely useful because they provide a means of linking an electrical signal with a very specific wavelength of visible light. It turns out that we can tailor these benefits by adding minute quantities of dopants to semiconductor materials. These are substances with at least one valence electron more or less than the semiconductor. Dopants are found in natural semiconductors, such as blue diamonds, but are also added synthetically.
If the dopant has one valence electron more than the pure semiconductor, excess electrons are available and conductivity improves. More specifically, less energy is required to excite an electron to the conduction band. This new crystal structure is known as a donor or n-type (negative) semiconductor.
If the dopant has one valence electron less than the pure semiconductor, its presence creates holes, or electron-attracting locations in the crystal. Again, this improves the conductivity and reduces the energy required to cross the band gap. The presence of the dopant creates an acceptor or p-type (positive) semiconductor.

The Hope Diamond contains trace amounts of boron and will conduct electricity, unlike a pure diamond, which is an insulator. Since the acceptor-level energy is so small, even the thermal energy at room temperature can produce this change, and the resulting holes in the valence band can move in the presence of an electric field.
The Hope Diamond
The Hope Diamond is one of the most striking examples of naturally doped semiconductors.
The Hope Diamond is the world’s largest deep blue diamond, with a cut weight of 45.52 carats. Formed deep in the Earth’s core over a billion years ago, it was brought to the surface by a diamond-bearing volcanic shaft in Golconda, India. Since its discovery in the early 1600s, it has crossed oceans and continents, and passed through the hands of kings and commoners. It has a notorious past, having been sold, stolen, recovered and re-cut from the French Blue diamond once owned by the French Royal Treasury.
The diamond takes its name from Henry Philip Hope, who added the blue diamond to his collection sometime between the death of King George IV in 1830, and 1839. Historians speculate that the King bought the diamond in the early 1800s, and that, following his death, it was sold to offset his debts. In 1958, Harry Winston, Inc. donated the Diamond the Smithsonian Institution, where it remains a compelling centerpiece of the National Museum of Natural History.