A page from the "Causes of Color" exhibit...
Why do lamps have differing shades of white?
Vapor lamps
Sodium vapor and mercury vapor lamps emit yellow and bright blue light, respectively. They are very efficient, and provide high-intensity light suitable for illuminating large, open areas. Mercury lamps also produce ultraviolet light; in fluorescent tube lamps, this is converted by fluorescence of a phosphor coating into lower-energy yellow, orange, and red light. This produces a more natural color balance for indoor lighting. Like compact fluorescent lamps, they are more energy-efficient than incandescent light bulbs.
A vapor lamp consists of a double tube. The vapor is contained in the internal tube, while the outer tube is kept empty of air, and absorbs dangerous high-energy ultraviolet radiation.
|
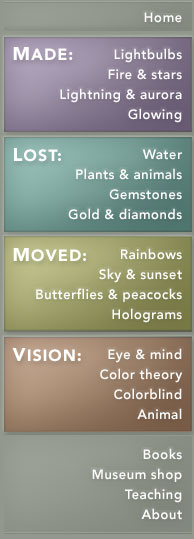
Energy-level scheme of an atom of sodium, showing some of the allowed transitions in returning back to the ground state after ionization. In a sodium vapor lamp, a high voltage produces ionization from the initial "ground state." As the electron escapes the atom, a sodium ion results. The electron returns to its ground state along specific allowed transitions, only a few of which are shown, emitting energy as radiation. Quantum theory defines the "selection rules" that determine this route. The lowest two arrows correspond to the emission of the bright-yellow sodium "doublet" lines at 589.7 (2.103 eV) and 589.0 nm (2.105 eV). |
Testing the inner tube of a mercury lamp at the Westinghouse Lamp Division, Bloomfield, N.J., in 1937. |
The table below compares the mechanism and energy efficiency of various lamps, as well as the colors produced by several of these lamps.
|
||||||||||||||||||||||||||||||||
- Initial Efficiency (Lumens per Watt). Lumens-per-watt (or lamp efficacy) ratings reflect only the watts consumed by the lamp itself and not ballast loss. Ballast is required in vapor lamps to limit the current that can flow, to stop the lamp from burning itself out.
Gas lasers, such as the helium-neon laser, use gas excitations with optical feedback from mirrors at each end of the tube. Lasers produce coherent light, in which all the light waves have almost the same frequency - they travel in the same direction and amplify each other.