A page from the "Causes of Color" exhibit...
The band structure of blue and yellow diamonds

The band structure of blue and yellow diamonds
A pure diamond crystal is translucent, as it is composed only of carbon atoms, each of which has four valence electrons.
In a yellow diamond, a few carbon atoms per million have been replaced by nitrogen atoms, each containing five valence electrons. The structure of the diamond crystal does not change significantly, but the extra electrons occupy a donor level.

Synthetic diamond crystals (3 mm across) grown at General Electric. The clear diamond is pure, the blue contains a boron acceptor, and the yellow contains a nitrogen donor. |
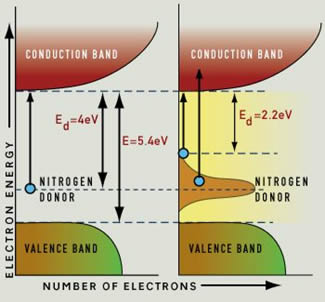
Electrons can be donated to the empty conduction band. The valence band is completely filled. At minute concentrations of nitrogen, the energy required to excite an electron from the donor level to the conduction band is 4 eV, an energy that is greater than the visible light range (left). The diamond will be colorless. At a few nitrogen atoms per million carbon atoms, the donor level is broadened as at the right of this figure, and energies greater than 2.2 eV can excite an electron from the donor level to the conduction band. The absorption of these higher energies (blue and violet light) results in the yellow color of the diamond. |
The nitrogen donor level energy in a diamond is large, peaking at about 4 eV. With a concentration of a few nitrogen atoms per million, instead of a clean "spike" donor level energy, the nitrogen donor level energy broadens into a band because of a number of complex factors, including thermal vibrations. This broadened donor energy band results in the difference between donor and conduction bands being as low as 2.2 eV. The most likely transition allows incident light with energy of 4 eV per photon to excite electrons from the donor level to the conduction band. However, it is possible for electrons to be excited to the conduction band with energy of 2.2 eV and upwards. This means that blue and violet light are absorbed from the full spectrum of light normally transmitted, and the resulting color is yellow.
Unlike blue boron-doped diamonds, which conduct electricity, nitrogen-doped diamonds remain insulators. This is because nitrogen is a deep impurity: a relatively high energy (2.2 eV, as compared with 0.4 eV for boron) is required to excite electrons from the donor energy level to the conduction band, and only a fraction of the available electrons are freed to carry a charge.
An extremely rare green color can result from a higher nitrogen content of about 1 atom per 1000 atoms of carbon. At even higher nitrogen concentrations, the donor level broadens so that all visible light can be absorbed, resulting in a black color.
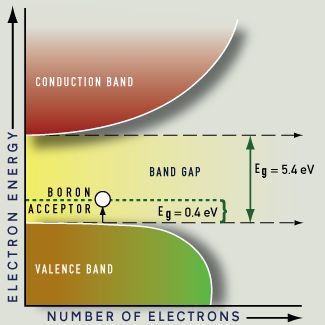
Synthetic blue diamonds are created by adding boron as an impurity. Boron is trivalent, having three valence electrons; where a boron atom replaces a carbon atom in the diamond structure there is one fewer electron than usual. This missing electron, or hole, creates an acceptor energy level above the valence band. The boron acceptor energy is only 0.4 eV, so light of any energy can be absorbed during excitation. The boron acceptor band is broadened, and the absorption tapers off throughout the visible light energies, resulting in stronger absorption at the red end of the spectrum. At a level of one or a few boron atoms for every million carbon atoms, an attractive blue color results. Natural diamonds of this color, such as the Hope Diamond, are rare and highly priced.