A page from the "Causes of Color" exhibit...
What is the role of chlorophyll?
Why do some plants appear green?
Green plants are green because they contain a pigment called chlorophyll. Chlorophyll absorbs certain wavelengths of light within the visible light spectrum. As shown in detail in the absorption spectra, chlorophyll absorbs light in the red (long wavelength) and the blue (short wavelength) regions of the visible light spectrum. Green light is not absorbed but reflected, making the plant appear green.
Chlorophyll is found in the chloroplasts of plants. There are various types of chlorophyll structures, but plants contain chlorophyll a and b. These two types of chlorophyll differ only slightly, in the composition of a single side chain.
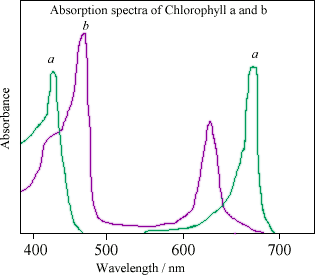
Absorption spectra showing how the different side chains in chlorophyll a and chlorophyll b result in slightly different absorptions of visible light. Light with a wavelength of 460 nm is not significantly absorbed by chlorophyll a, but will instead be captured by chlorophyll b, which absorbs strongly at that wavelength. The two kinds of chlorophyll in plants complement each other in absorbing sunlight. Plants are able to satisfy their energy requirements by absorbing light from the blue and red parts of the spectrum. However, there is still a large spectral region between 500 and 600 nm where chlorophyll absorbs very little light, and plants appear green because this light is reflected.
What is chlorophyll?
Chlorophyll is a compound that is known as a chelate. A chelate consists of a central metal ion bonded to a large organic molecule, composed of carbon, hydrogen, and other elements such as oxygen and nitrogen.
Chlorophyll has magnesium as its central metal ion, and the large organic molecule to which it bonds is known as a porphyrin. The porphyrin contains four nitrogen atoms bonded to the magnesium ion in a square planar arrangement. Chlorophyll occurs in a variety of forms.
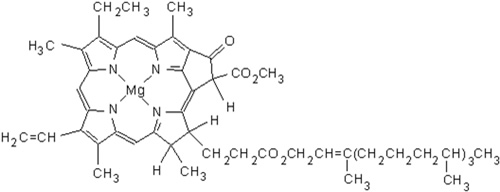
The structure of chlorophyll a.
Chlorophyll does not contain chlorine as the name might suggest; the chloro- portion stems from the Greek chloros, which means yellowish green. The element chlorine derives its name from the same source, being a yellowish-green gas.
How do birds and animals see plants?
Vegetation will not appear to animals as it does to us. Although our color perception is the most advanced amongst mammals, humans have less effective color vision than many birds, reptiles, insects and even fish. Humans are trichromats, sensitive to three fundamental wavelengths of visible light. Our brains interpret color depending on the ratio of red, green and blue light. Some insects are able to see ultraviolet light. Birds are tetrachromatic, able to distinguish four basic wavelengths of light, sometimes ranging into ultraviolet wavelengths, giving them a far more sensitive color perception.
It is hard for us to imagine how the world appears to birds, but they will certainly be able to distinguish more hues of green than we do, and so are far more able to distinguish between types of plants. We can speculate that this is of great benefit when choosing where to feed, take shelter and rear young. Aquatic creatures, from fish to the hyperspectral mantis shrimp (which distinguishes up to twelve distinct wavelengths of light) are uniquely tuned to the colors of their environment. The pages on animals include more information on the variety of color vision in the animal kingdom.
The vivid colors of fall leaves emerge as yellow and red pigments, usually masked by chlorophyll, are revealed by its absence. Chlorophyll decomposes in bright sunlight, and plants constantly synthesize chlorophyll to replenish it. In the fall, as part of their preparation for winter, deciduous plants stop producing chlorophyll. Our eyes are tuned to distinguish the changing colors of the plants, which provide us with information such as when fruits are ripe and when the seasons are starting to change.
Apart from coloring, has chlorophyll any other role?
The green color of chlorophyll is secondary to its importance in nature as one of the most fundamentally useful chelates. It channels the energy of sunlight into chemical energy, converting it through the process of photosynthesis. In photosynthesis, chlorophyll absorbs energy to transform carbon dioxide and water into carbohydrates and oxygen. This is the process that converts solar energy to a form that can be utilized by plants, and by the animals that eat them, to form the foundation of the food chain.
Chlorophyll is a molecule that traps light - and is called a photoreceptor.
Photosynthesis
Photosynthesis is the reaction that takes place between carbon dioxide and water, catalysed by sunlight, to produce glucose and a waste product, oxygen. The chemical equation is as follows:

Glucose can be used immediately to provide energy for metabolism or growth, or stored for use later by being converted to a starch polymer. The by-product oxygen is released into the air, and breathed in by plants and animals during respiration. Plants perform a vital role in replenishing the oxygen level in the atmosphere.
In photosynthesis, electrons are transferred from water to carbon dioxide in a reduction process. Chlorophyll assists in this process by trapping solar energy. When chlorophyll absorbs energy from sunlight, an electron in the chlorophyll molecule is excited from a lower to a higher energy state. The excited electron is more easily transferred to another molecule. A chain of electron-transfer steps follows, ending when an electron is transferred to a carbon dioxide molecule. The original chlorophyll molecule is able to accept a new electron from another molecule. This ends a process that began with the removal of an electron from a water molecule. The oxidation-reduction reaction between carbon dioxide and water known as photosynthesis relies on the aid of chlorophyll.

There are actually several types of chlorophyll, but all land plants contain chlorophyll a and b. These 2 types of chlorophyll are identical in composition apart from one side chain, composed of a -CH3 in chlorophyll a, while in chlorophyll b it is -CHO. Both consist of a very stable network of alternating single and double bonds, a structure that allows the orbitals to delocalize, making them excellent photoreceptors. The delocalised polyenes have very strong absorption bands in the visible light spectrum, making them ideal for the absorption of solar energy. |

The chlorophyll molecule is highly effective in absorbing sunlight, but in order to synthesize carbohydrates most efficiently, it needs to be attached to the backbone of a complex protein. This protein provides exactly the required orientation of the chlorophyll molecules, keeping them in the optimal position that enables them to react efficiently with nearby CO2 and H2O molecules. This bacterial photoreceptor protein forms the backbone for a number of chlorophyll molecules. |
The basic structure seen in the chlorophyll molecule recurs in a number of molecules that assist in biochemical oxidation-reduction reactions, because it is ideally suited to promote electron transfer. Heme consists of a porphyrin similar to that in chlorophyll with an iron (II) ion at its center. Heme is bright red, the pigment that characterizes red blood. In the red blood cells of vertebrates, heme is bound to proteins to form hemoglobin. Oxygen enters the bloodstream in the lungs, gills or other respiratory surfaces and combines with hemoglobin. This oxygen is carried round the body of the organism in the bloodstream and released in the tissues. Hemoglobin in the muscle cells is known as myoglobin, a form that enables the organism to store oxygen as an electron source, ready for energy-releasing oxidation-reduction reactions.
Commercial pigments
Chlorophyll is a pigment that causes a green colour. Chlorophyll as a green dye has been used commercially in processed foods, toothpaste, soaps and cosmetics. Commercial pigments with structures similar to chlorophyll have been produced in a range of colors. In some, the porphyrin is modified, for example by replacing the chlorine atoms with hydrogen atoms. In others, different metal ions may be present. Phthalocyanine is a popular bright blue pigment with a copper ion at the center of the porphyrin.

Phthalocyanine is a blue pigment.