A page from the "Causes of Color" exhibit...
Iodine and starch

The phenomenon of charge transfer is seen in gemstones such as blue sapphires. You can try out this process yourself using iodine and starch. Iodine produces a charge-transfer complex with starch, producing an intense color.
The starch test
Many different food groups contain a carbohydrate known as starch. Using an iodine solution, you can test for the presence of starch. When starch is present, the iodine changes from brown to blue-black or purple.
Warning
Be careful in handling iodine. It can stain clothing, equipment and skin. DO NOT put iodine in your mouth and DO NOT eat any tested foods, as iodine can be poisonous. Wash your hands and throw everything away when done.
You will need:
- Dropper or syringe
- Iodine disinfectant that you can purchase from a pharmacy or chemist. You can use Betadine (a povidone-iodine mixture), Lugol’s solution (an iodine-potassium mixture), or tincture of iodine (here the iodine is dissolved in alcohol, or alcohol and water), depending what is available. They all have a very strong color, so dilute the mixture with about 10 parts water to see the reaction more clearly.
- Starchy solution, such as corn starch (cornflour) in water
- Non-starchy solution, such as milk, for comparison
- Starchy foods to test: potato (cooked or raw), pasta, rice, or bread
- Non-starchy foods for comparison: apple, cucumber, pure sugar (the other main category of carbohydrate), and any others you would like to try
- Disposable plastic cups or containers
- Newspaper
- Paper plates
- Paper towels
Method
- Cover your working surface with newspaper.
- Place the paper plates on the newspaper.
- Place the cups on top of the paper plates.
- Put different food solutions in each cup, e.g. corn starch in water, flour in water, milk, and water.
- Using the dropper, add a drop or two of the iodine solution to each cup.
- Place a slice of a potato on a paper towel atop a paper plate and add a drop of the iodine solution to the potato slice. Note the color change.
- Repeat with the variety of starchy and non-starchy foods you have selected.
- Make a chart up with columns like the one below. Fill out and have fun!
- When you’re done, wash your hands and throw everything away.
| Food | Color change | Is starch present? |
|---|---|---|
A change of color to a blue-black or purple color suggests that starch is present. If there is no change in color, this suggests no starch is detectable
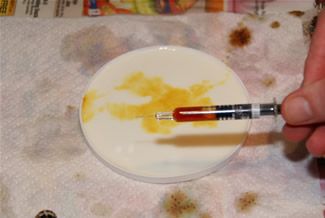
Note the change of the iodine to black on the potato (right) but not the apple (left). (10% Tincture of iodine.) Potato contains starch, but apple does not.
|
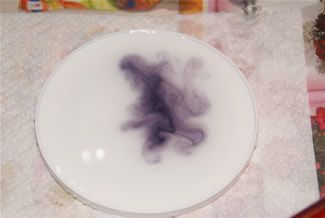
A solution of corn starch in water (left) turns purple while the milk (right) remains unchanged. (10% Tincture of iodine.) Milk has no starch. |
|
|
Cooked rice changes color, testing positive for starch. (10% Tincture of iodine.) |
||
Why does it work?
Starch is a carbohydrate and exists in two types of molecules: amylose (linear) and amylopectin (branched). Most starch contains a mixture of these two molecules, generally with more amylopectin (65% to 85%). The reaction between amylose (even though it is often present in lesser amounts) and iodine is said to account for the intense color change seen.
Many details of the reaction of iodine with starch are unknown, but one explanation is that when a solution of diluted iodine is added to starch, an intensely colored starch-iodine complex forms.
Amylose molecules consist of single, mostly unbranched chains of glucose molecules, shaped like a spring. It is speculated that the iodine (in the form of I5- ions) gets stuck in the coils of the beta amylose molecules (soluble starch). The starch forces the iodine into a linear arrangement in the middle groove of the amylose coil. There is some transfer of charge between the starch and the iodine. This changes the electron arrangements and hence the spacings between energy levels. The new spacings absorb visible light selectively and give the complex its intense blue color.
Note: The effect is only seen when both iodine as an element and iodide as an ion are present. Iodine is not very soluble in water and the addition of iodide makes it soluble. Iodine, together with the iodide ion, forms a complex which dissolves in water, unlike iodine on its own. Molecular iodine (I2) reacts with iodide (I-) which is a negatively charged ion and creates an anion (I3-). The anion dissolves easily in water (which is polar). There is still some dispute about the exact mechanism involved in producing the unmistakable color change, but this charge transfer process is widely accepted as the most likely.
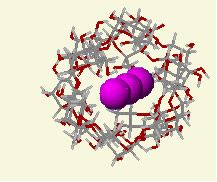
Iodine inside amylose resulting in a blue-black color
Amylopectin, having a branching structure, reacts with iodine to form a reddish brown or purple solution. Since amylopectin is highly branched, it only binds a small amount of iodine and produces a paler purple-red color. The ratio of amylose to amylopectin varies according to the type of food.
More ideas
Foods that are high in starch include grain foods and some vegetables, such as dried beans and peas, potatoes, yams, and corn. As fruits ripen, the amount of starch can decrease. Unripe bananas contain a fair amount of starch, but ripe bananas don’t, as the starches have transformed to sugars. A ripe banana will not produce a bluish-black color with iodine, but you will see the effect in a greener banana. You can experiment to see how the amount of starch has changed.
The starch test is used in industry. In beer brewing, a negative starch test result confirms that all the starches in the beer have been converted to sugars, as expected.