A page from the "Causes of Color" exhibit...
Why are rubies red?
Red ruby. The name ruby comes from the Latin "Rubrum," meaning red. The ruby, along with the sapphire, is in the corundum group. Rubies are made of corundum with chromium present as an impurity. The brightest red - and thus most valuable - rubies are usually from Burma. Dark and violet red rubies come principally from Thailand. Small quantities of rubies are also found in Sri Lanka, Cambodia, Pakistan, India, and Tanzania. Rubies have long been cherished among the world’s most beautiful and valuable gems. The hardest mineral after the diamond, the ruby’s brittleness requires care when cutting.
Why are rubies red?
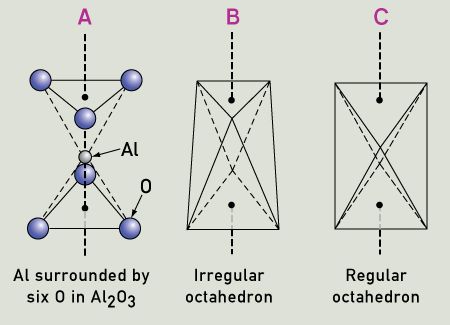
A crystal of pure Al2O3 is known as corundum, and as colorless sapphire when in gem-quality form. In this material, six oxygen atoms surround each aluminum atom in the form of a slightly distorted octahedron.
The three oxygen atoms above the aluminum are closer to each other than the three oxygen atoms below, and the aluminum atom is a little lower than halfway down. Half of the aluminum atoms have this arrangement, and the other half have an inverted arrangement. If this arrangement is viewed in terms of ionic bonds, then the positive aluminum ion is surrounded by six negative charges (oxygen ions). Each aluminum atom donates three electrons to become Al3+ and has no unoccupied energy levels, while each oxygen atom receives two electrons, ensuring that it has no unoccupied energy levels. Therefore, two aluminum atoms donate a total of six electrons, and three oxygen atoms receive a total of six electrons, to produce Al2O3. They produce an electrostatic field around the aluminum ions, called a crystal field. Since the bond is not purely ionic, but has some covalent nature, it can also be treated as a ligand field. So, not only the electric charges, but also the specific bonding characteristics of the ligands (ions or molecules surrounding the central atom or ion) are included. The symmetry and strength of the ligand field both need to be considered when predicting the effects of the ligands.
In pure corundum, all electrons are paired and there is no absorption of light. Once one out of every hundred aluminum atoms is replaced by chromium atoms, negatively charged oxygen ions surround the aluminum ion (which has donated 3 electrons), so a chromium atom must donate three electrons to become Cr3+ , replacing Al3+, in order for the charge to remain the same. In Al3+ there are no partially filled energy levels or orbitals. However, in Cr3+ there are partially filled energy levels or orbitals. It is these electrons that can be excited and that cause absorption of certain wavelengths of light, resulting in color.
The distorted-octahedral oxygen-ligand environment around an Al ion in corundum Al2O3 is shown in two different ways, (A) and (B), in comparison with a regular octahedron (C).
Orbitals have specific shapes and geometric configurations in space. In Cr3+ , there are three unpaired electrons in the outer energy level (which we will call 3d). This energy level can accommodate a total of 10 electrons, so there are unoccupied energy levels. So in this case there are three out of five orbitals partially filled. In an isolated Cr3+ ion, the fact that the five 3d orbitals point in different directions does not matter, and it would be colorless. However, in a ruby, the five 3d orbitals of the Cr3+ ion are surrounded by six oxygen ions in a distorted octahedral configuration. Interactions with the orbitals of the six oxygen ligands produce shifts in the energy levels of the individual orbitals.
The five 3d orbitals of the Cr3+ ion no longer have the same energy. The splitting between these energy levels is determined by symmetry. Arranging the ligands octahedrally or tetrahedrally will produce different shifts in energy.
Light in the form of a photon can be absorbed if the energy of the photon exactly matches the energy needed for an electron to "jump" to a higher energy level. For energy to be conserved, the photon energy must match the "jump" energy, which is the difference in energy between the final state and the initial state of the electron.
The splitting of the five 3d orbitals in a tetrahedral and an octahedral ligand field. The five equal energy d-orbitals of a Cr3+ ion are shown in the center. Such energy levels, however, are perturbed by the existence of the six neighboring oxygen ions - the "ligands." Both the geometrical distribution (octahedral, tetrahedral, etc.) and the strength of the bonding (or the equivalent size of the electric field produced by the oxygen ions - the "crystal field" or "ligand field") affect the distribution and spacing of the levels.
The term diagram (allowed transitions between the energy levels of atoms) of Cr3+ in a distorted octahedral field (A), the energy levels and transitions in ruby (B), and the resulting absorption spectrum and fluorescence of ruby (C) and emerald (D). The symmetry of the ligand field and its strength combine to give the energy level scheme, the "term diagram," and the transition scheme. Here the violet and yellow-green regions of light passing through ruby are absorbed, as in the two upward arrows leading to the 4T1 and 4T2 levels, thus producing the absorption spectrum shown at C. Selection rules don’t permit absorption to the 2E level. Red light is transmitted and there is a small blue transmission, giving ruby its deep red color with purple (bluish) overtones. The selection rules do not permit a return from the two excited states (4T1 and 4T2) directly to the ground state. In losing its energy again, the Cr3+ must pass through the state labeled 2E with the emission of some heat. In returning from 2E back down to the ground state, a quantum of red light, the red fluorescence of ruby, is emitted. This is best seen under ultraviolet illumination. The absorptions in emerald will be discussed later.